bcl3 polar or nonpolar
Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie. That means the angle between each of the bonds is 120.
 |
| Vespr Theory Ppt Download |
The electronegativity difference ΔEN 316 296 02.
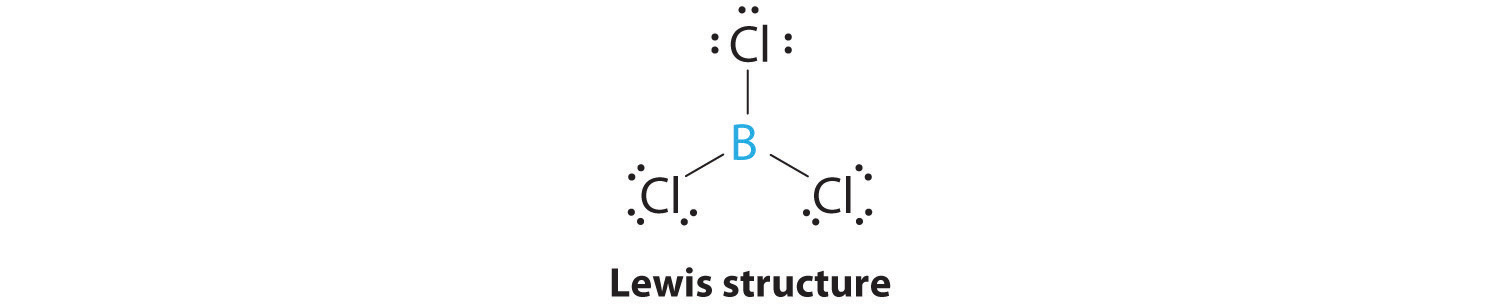
. Is the molecule BCl3 polar or nonpolar. The B-Cl bonds are polar but the molecule is not. If BCl3 is a Non-polar Molecule Why Does It Form Polar Bonds. Let me explain this to you in 3 steps.
Hence each C-H bond is a nonpolar covalent bond. The electronegativity difference ΔEN 316 219 097 This value lies between. Therefor it is a non polar compound. Is citric acid very polar moderately polar or nonpolar.
Of the molecules below only is nonpolar. BCl3 is a nonpolar molecule. Yes it is non polar. BCl3 H2O NH3 and PCl3 which is non-polar.
This value lies between 04 to 20. A BF3 b H2O c NH3 d HCl e. To have a polar bond it needs to have an asymmetrical shapeshift in electron density to form an electrical dipole but this is not the case. Learn to determine if BCl3 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then us.
Is BCl3 a polar bond. BCl3 is a nonpolar molecule because it does not have any pole of positive charge and negative charge on it. If the electronegativity difference ΔEN is. This value is less than 04 which indicates that the bond between Bromine Br and Chlorine Cl is nonpolar.
Is BCl3 polar or non-polar. Have a look at the above image. Electronegativity of Chlorine Cl 316 Now lets see the polarity of each bond. The B-Cl bond itself is polar because of the difference in.
The electronegativity difference ΔEN 316 255 061. Answer 1 of 3. While it is true that BCl3 is non-polar it forms bonds because the B-Cl. The reason why this is.
NCl_3 has the same geometry as ammonia which is pyramidal with a C_3v point group. The B-Cl bond itself is polar because. Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie. Is the compound C_2H_3Cl polar or nonpolar and why.
1 There is a net dipole moment but it is very weak since. The shape of BCl3 is triangular. Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie. Boron trichloride BCl3 is a nonpolar molecule because chlorines halides are symmetrically located around the central boron atom which cancels out polar covalent bonds.
HttpsshortlyimB8Ewq The BCl3 molecule is known to be trigonal planar. You should review shapes of molecules. If the electronegativity difference ΔEN is less than 04 then the bond is nonpolar covalent bond. The B-Cl bond itself is polar because of the difference in.
 |
| Contoh Senyawa Ikatan Polar Dan Non Polar Cara Praktikumnya Youtube |
 |
| Is Bcl3 Polar Or Nonpolar Techiescientist |
 |
| Solved Which Is Polar Among Bcl3 Fcl5 Ch4 And Scl6 Course Hero |
 |
| Is Bcl3 Polar Or Non Polar Boron Trichloride Youtube |
 |
| Solved What Is The Molecular Geometry And The Polarity Of Chegg Com |
Posting Komentar untuk "bcl3 polar or nonpolar"